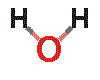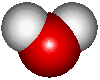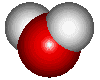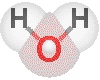
I thought a diagram of water may help to understand it's greenhouse gas properties.
 Remember, weather is always changing, but, the climate is suppose to remain stable. This episode of warming is dangerous because it is abrupt climate change. It may not seem abrupt because it is taking place over 150 years, but, on Earth's timeline that is a very short time. Abrupt climate change destroys life as it cannot adapt in such short periods of time. This is also different in that the source of greenhouse gases seem infinite. Infinite sources of greenhouse gases makes the final outcome of Earth unknown. The BURNING and manufacture of these gases is a far different method of Earth's experience with greenhouse gases before the Industrial Revolution.
Remember, weather is always changing, but, the climate is suppose to remain stable. This episode of warming is dangerous because it is abrupt climate change. It may not seem abrupt because it is taking place over 150 years, but, on Earth's timeline that is a very short time. Abrupt climate change destroys life as it cannot adapt in such short periods of time. This is also different in that the source of greenhouse gases seem infinite. Infinite sources of greenhouse gases makes the final outcome of Earth unknown. The BURNING and manufacture of these gases is a far different method of Earth's experience with greenhouse gases before the Industrial Revolution.
The other greenhouse gases are very stable molecules. While water is a stable molecule, it is also highly reactive as a partially charged structure.
Water has a bent structure as noted below. It has to hydrogens that are bonded to a single oxygen through shared electrons as noted above. The bend in the molecule allows for water to have a PARTIAL positive charge on the oxygen side and a PARTIAL negative charge on the hydrogen side. This partial positive or negative provides enough reason for other molecules to interact with it.
The point is water is a greenhouse gas because it can mitigate the heat. As the other greenhouse gases turn photon light from the sun to infrared warming heat from Earth's surface, water is available to receive that heat and become weather.
 Remember, weather is always changing, but, the climate is suppose to remain stable. This episode of warming is dangerous because it is abrupt climate change. It may not seem abrupt because it is taking place over 150 years, but, on Earth's timeline that is a very short time. Abrupt climate change destroys life as it cannot adapt in such short periods of time. This is also different in that the source of greenhouse gases seem infinite. Infinite sources of greenhouse gases makes the final outcome of Earth unknown. The BURNING and manufacture of these gases is a far different method of Earth's experience with greenhouse gases before the Industrial Revolution.
Remember, weather is always changing, but, the climate is suppose to remain stable. This episode of warming is dangerous because it is abrupt climate change. It may not seem abrupt because it is taking place over 150 years, but, on Earth's timeline that is a very short time. Abrupt climate change destroys life as it cannot adapt in such short periods of time. This is also different in that the source of greenhouse gases seem infinite. Infinite sources of greenhouse gases makes the final outcome of Earth unknown. The BURNING and manufacture of these gases is a far different method of Earth's experience with greenhouse gases before the Industrial Revolution.
But, as to water, for all it's incredible properties that keeps life happening in Earth, it's property of 'temperature-phase' change is where it fits into this classification as a greenhouse gas.
